Standardized terminologies in healthcare allow for the exchange of patient data and information, which is the foundation of interoperability. To identify textual data, each data element needs to be linked to a unique code that translates it. Standardized vocabularies, like LOINC and SNOMED CT, are used to distinguish diagnostic terms and are developed by recognized groups of experts. Standard vocabularies, particularly coded laboratory results, enable automated decision support for better patient care, and automated public health surveillance.
LOINC
Logical Observation Identifiers Names and Codes (LOINC®) is one of several standards used to exchange clinical health information. LOINC is the preferred standard of HL7 International for identifying laboratory tests. LOINC codes embedded in HL7 messages allow healthcare facilities that receive messages from multiple sources to electronically file those results into the correct slots in the EHR or other information system. This communication allows broad distribution of healthcare information without the need for individual institutions to exchange master files for data, such as test or result codes. Each institution maps its own local vocabularies to the standard code, allowing data to be shared broadly, rather than remaining isolated as a single island of information.
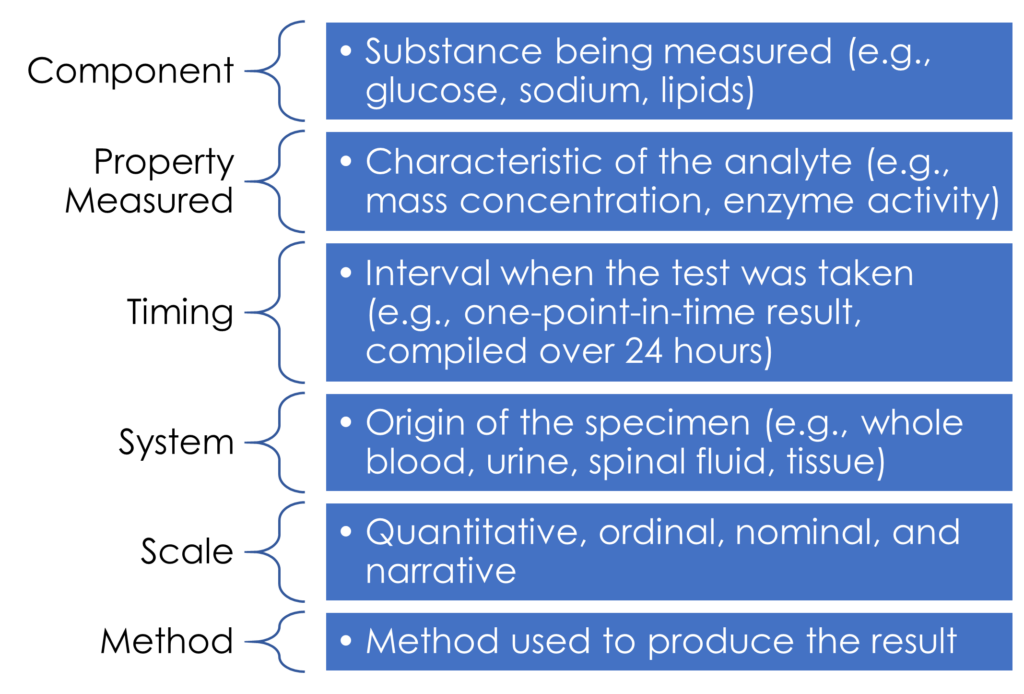
LOINC was created in 1994 by the Regenstrief Institute Inc., in response to a demand for the electronic transmission of clinical data. LOINC assigns identifying codes to more than 70,000 medical terms to enable transmission into an EHR. Because of the extensive number of codes, a program called Regenstrief LOINC Mapping Assistant (RELMA®) assists with LOINC mapping. Both LOINC and RELMA are downloadable from the LOINC website. LOINC codes contain six parts in a specific order, each with a discrete meaning that together describe in detail how a particular lab test is performed (see Figure 1).
The purpose of the LOINC database is to provide universal identifiers for observations in HL7 messages. In the setup of HL7, one field (OBX-3) holds the observation identifier, and another field (OBX-5) holds the observation value. Specifically, LOINC provides a standard code system for the observation identifier field (OBX-3). Prior to LOINC adoption, laboratories sent their own internal identifying codes in the OBX-3 field of the HL7 interface message. However, laboratories had unique test codes, which made data sharing and interfacing more difficult.
Large healthcare institutions have taken advantage of LOINC to standardize the information coming from many different sources. The use of LOINC codes to identify laboratory tests can provide major benefits to organizations that receive such messages because it allows them to organize and analyze results from many HL7 sources. Healthcare organizations can import test results into their EHR in a codified manner when their source laboratories include LOINC codes in their HL7 messages. As a result, LOINC aids in data interoperability. The granularity inherent in LOINC ensures accurate comparison of data from multiple, disparate sources.
Standards like LOINC are imperative in improving patient care and reducing the costs associated with redundant and duplicative testing. Greater interoperability of lab results means that organizations can aggregate larger volumes of data for individual patients to support population health management. The ability to aggregate this data in the same language allows providers to analyze and report their patient care performance more easily and accurately. By fostering this kind of interoperability, LOINC can help healthcare organizations achieve better patient care and improved revenue.
SNOMED CT
Systemized Nomenclature of Medicine – Clinical Terms (SNOMED CT®) is a clinical terminology that provides clinical content for medical documentation with greater granularity than LOINC, and must be used when describing more complicated, process-driven tests, such as anatomic pathology and microbiology.
LOINC Combined with SNOMED
So how do LOINC and SNOMED work together and why do laboratories need both? An easy way to think about it is that LOINC provides codes that ask the question, and when needed, SNOMED provides codes for the answer. LOINC includes codes that identify the test observation (e.g., serum glucose or blood culture), not the codes that might be reported in the values of those test observations. LOINC is used to code the testing method, whereas SNOMED CT codes for non-numeric answers.
In HL7 messages, LOINC provides codes for the question in fields OBR-4 and OBX-3, while SNOMED provides codes for the answers in OBX-5. For more complex test results, SNOMED CT is needed to properly define testing.
A common interaction between LOINC and SNOMED CT occurs in microbiology. For example, when a culture is ordered, that order will be coded in LOINC. However, the most sophisticated communication LOINC can offer for the results would be the general microbe physical description (e.g., gram-negative cocci). The non-numerical result would be coded in SNOMED CT. SNOMED CT can take the result of the complex biogram, the organism’s name, and create the binary code to communicate it across an interface. The gram-negative cocci can then be fully identified.
Because of this connection between the two coding systems, there is an initiative by the National Library of Medicine and Regenstrief Institute currently under way to map LOINC to SNOMED CT for a closer integration between the two terminologies.
LOINC & SNOMED Join to Address Pandemic
The Regenstrief Institute and SNOMED International are collaborating to help manage the SARS-CoV-2 public health emergency. These organizations have teamed up to code and track SARS-CoV-2 testing and COVID-19 cases to help contain the virus. Because both of these standards are already commonly in place in our healthcare system, their actions are quickly enhancing efforts to address the pandemic.