 Laboratory competency assessments are important to ensure that lab testing is being performed accurately. However, among all laboratory inspection agencies — Clinical Laboratory Improvement Amendments (CLIA), College of American Pathology (CAP), Commission on Office Laboratory Accreditation (COLA), Joint Commission, etc. — the most cited deficiency is related to incomplete competency assessment performance or documentation.1,2 This has been the top regulatory deficiency for years because it is an enormous and complex task.
Laboratory competency assessments are important to ensure that lab testing is being performed accurately. However, among all laboratory inspection agencies — Clinical Laboratory Improvement Amendments (CLIA), College of American Pathology (CAP), Commission on Office Laboratory Accreditation (COLA), Joint Commission, etc. — the most cited deficiency is related to incomplete competency assessment performance or documentation.1,2 This has been the top regulatory deficiency for years because it is an enormous and complex task.
With hundreds or thousands of end users for point-of-care testing (POCT), and dozens of devices and methodologies, performance and documentation of personnel competency assessments can be daunting. To stay compliant and ensure accurate test results, laboratories need effective data management software that can track, automate, and document as much of the assessment process as possible.
Executive Summary
- Laboratory competency assessments are important to ensure that lab testing is being performed accurately.
- The most common deficiencies cited during laboratory inspection are for incomplete competency assessment performance or documentation.
- Competency assessments and training are not the same. Training occurs before testing; competency is ongoing after initial training.
- Competency assessment requirements are part of the personnel portion of a Quality Management System (QMS).
- Competency assessments have six components, as well as guidelines for who can evaluate and how often they are required.
- Laboratories can benefit from data management software that can track and automate as much of the assessment process as possible to document compliance.
What Are Laboratory Personnel Competency Assessments?
Competency assessments are used to determine the ability of personnel to apply their skills, knowledge, and experience to perform their laboratory duties correctly so that accurate lab results are achieved and reported. Competency assessments help ensure that laboratory personnel are proficient in performing a specific test and can fulfill their duties as required by federal regulations.
Initial training of how to perform a test is not considered a competency assessment. Training must take place prior to assessment of personnel competency.
Why Are Competency Assessments Important?
Though complicated and time-consuming, an effective competency assessment program works to minimize and prevent errors, standardize processes, improve efficiency, and
ensure patient safety through consistent monitoring of laboratory practices. Competency assessments are part of the laboratory’s QMS, which is a CLIA requirement for maintaining laboratory accreditation.
Implementing a QMS enables laboratories to operate more efficiently by reducing nonconformance. A QMS encompasses all aspects of a laboratory’s operations, including
procedures, documentation, personnel training, quality control practices, and continuous improvement processes, which are necessary for maintaining consistent quality. The
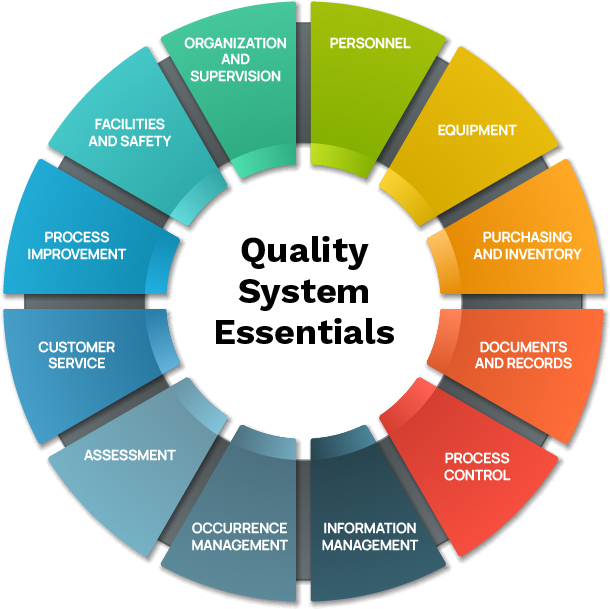
Clinical and Laboratory Standards Institute (CLSI) defines the 12 quality system essentials necessary for a strong laboratory QMS, which includes personnel. See Figure 1.
The CLSI guideline, A Quality Management System Model for Laboratory Services, helps laboratories implement and maintain an effective QMS. The personnel guidance states
that labs must staff an adequate number of trained employees who are competent to perform their duties. The requirements include job qualifications, job descriptions, training,
performance appraisals, competency assessments, and continuing education. Training and competency assessments are required at the beginning of employment and regularly
thereafter to ensure staff remain competent to carry out tasks as expected. Ongoing assessment is required to verify job performance, separate from initial training.
What Are The Competency Assessment Regulatory Requirements?
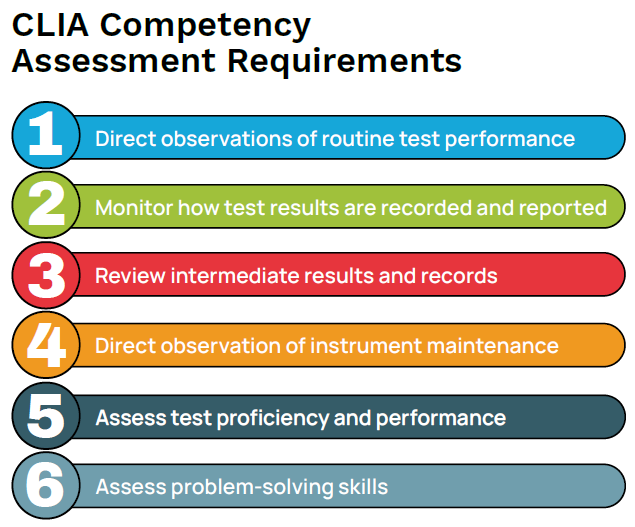
CLIA requirements for competency assessments include six procedures for all personnel performing laboratory testing.
- Direct observations of routine patient test performance, including patient preparation (if applicable), specimen handling, processing, and testing
- Monitoring the recording and reporting of test results
- Review of intermediate test results or worksheets, quality control records, proficiency testing results, and preventive maintenance records
- Direct observations of instrument maintenance and function checks
- Assessment of test performance through testing previously analyzed specimens, internal blind testing samples, or external proficiency testing samples
- Assessment of problem-solving skills3
 All six procedures must be performed for testing personnel for each test that the individual is approved by the laboratory director to perform. State requirements may be more stringent (e.g., New York requires compliance with safety protocols and assessment of delegated supervisory functions). A robust laboratory information system can help your lab address each of these procedures in targeted ways.
All six procedures must be performed for testing personnel for each test that the individual is approved by the laboratory director to perform. State requirements may be more stringent (e.g., New York requires compliance with safety protocols and assessment of delegated supervisory functions). A robust laboratory information system can help your lab address each of these procedures in targeted ways.
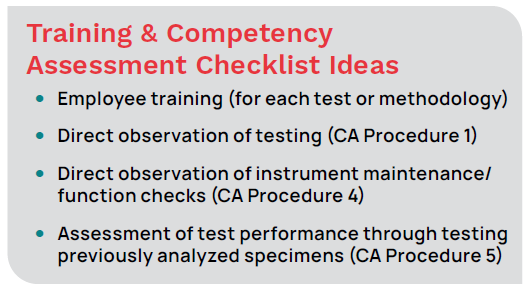
For the first procedure — direct observations of routine patient test performance — employees are observed while performing the test, including patient preparation, specimen handling, processing, and testing. This is an example of an opportunity to use a checklist to document that all steps are completed. Findings from procedures two and three—recording and reporting of test results and review of test results or worksheets, quality control records, proficiency testing results, and preventive maintenance records can also be recorded on a checklist or elsewhere in the POCT data management solution.
For the second procedure — monitoring the recording of test results — assessors can review worksheets or manual test logs to compare to final reports, evaluate turnaround times, and evaluate adherence to procedures.
For the fourth and fifth procedures — direct observations of instrument maintenance and assessment of test performance — you can use checklists to document instrument performance and maintenance and function checks for adherence to procedure. Checklists are also helpful when documenting running a test with known results (blind or proficiency testing).
For the sixth procedure—assessment of problem-solving skills—assessors can review problem logs, incident reports, QC failures, and corrective actions. Case studies with
quizzes that cover pre-analytic, analytical, and post-analytic processes are helpful tools to assess an employee’s problem-solving skills.
Who Is Required To Have A Competency Assessment?
Competency is required for all technical, supervisory, and testing personnel. This includes CLIA clinical consultants, technical consultants, technical supervisors, general supervisors, and testing personnel. Clinical consultants, technical consultants, technical supervisors, and general supervisors who perform patient testing must also have the six required procedures in their competency assessment in addition to a competency assessment based on their federal regulatory responsibilities.3
Who Can Perform Competency Assessments?
For moderate-complexity testing, competency assessments are performed by the technical consultant or other personnel who meet the regulatory qualification requirements for
technical consultant.
For high-complexity testing, the technical supervisor should perform the competency assessments (CA), or they can be delegated, in writing, to a qualified general supervisor. Peer-testing personnel who do not meet the regulatory qualifications of a technical consultant, technical supervisor, or general supervisor (as defined by CLIA) cannot perform competency assessments. Ultimately, it is the responsibility of the laboratory director to ensure that all testing personnel are competent and maintain their competency to perform and report accurate and reliable test results.
 How Often Are Competency Assessments Required?
How Often Are Competency Assessments Required?
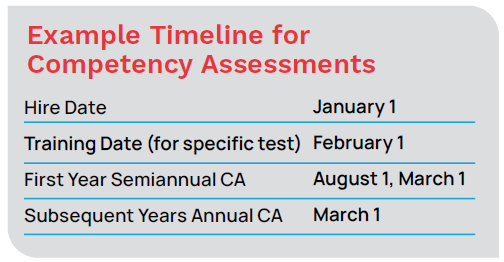
Prior to performing patient testing, training must be completed and evaluated for proper test performance. Separate from training, personnel competency assessments are required at least semiannually during the first year and annually thereafter. Laboratories can incorporate competency evaluations throughout the year by coordinating them with routine practices and procedures to minimize impact on workload.
Laboratories must have a plan to address areas where personnel do not demonstrate competence. If there is a problem during review (e.g., results do not match), you must complete a competency corrective action report that may require retraining and reassessing the employee.
 Are Competency Assessments Required For Waived Testing?
Are Competency Assessments Required For Waived Testing?
POCT can be waived or nonwaived. CLIA does not require competency assessments for waived testing. However, labs must still ensure that patient testing results are correct to assist in making an accurate patient diagnosis. For waived POCT, performing competency testing is considered good laboratory practice.
For nonwaived or moderately complex POCT, competency assessments must be performed and documented. All six CLIA evaluation areas must be included for every testing personnel, for each test performed.
 How Can Your POCT Data Management System Help?
How Can Your POCT Data Management System Help?
A POCT program requires a plan to ensure that each person performing POCT maintains satisfactory levels of competence for each test system across all end users. The lab’s
QMS defines specific personnel requirements, and CMS further defines specific competency evaluation criteria. For large organizations testing across multiple locations, this can be a tremendous POCT management challenge. This complex scenario is why a POCT-focused data management system can offer enormous benefit. For example, you can create a training checklist in your POCT data management solution.
A POCT data management solution can help track and document all aspects of competency assessments and have that information readily retrievable. The POCT solution can autodeliver quizzes that are custom-made for each test and track due dates for all end users.
Orchard® Point-of-Care™
Orchard® Software offers Orchard® Point-of-Care™, an advanced POCT data management system that enables point-of-care coordinators to track patient testing, devices, operator certifications, and QC from a central location to help ensure quality testing and promote patient safety.
Orchard® Competency Module
Alongside Orchard Point-of-Care, the Orchard® Competency Module helps lab managers and point-of-care coordinators create, assess, and track training and competency evaluations for laboratory personnel and POCT end users. The module can be used to develop and autodeliver custom quizzes to ensure that training and competency requirements are met, and lab testing is being performed by certified individuals.
Orchard Point-of-Care and Orchard Competency Module can help:
- Ensure that requirements are met and lab testing is being performed by certified individuals
- Track training and competency evaluations due dates for POCT operators in all locations across the organization
- Auto-calculate certification expiration dates and notify operators of upcoming due dates
- Include notes for training and scanned competency assessments
- Make bulk changes (e.g., recertify all staff selected for a specific date)
- Associate certified personnel with specific devices and locations
- Allow an operator to only perform certain order choices on a specific instrument type
- Restrict operators who are not up to date on their certification
- Auto-deliver custom quizzes
- Improve efficiency of lab personnel certifications with custom checklists, operator auto-recertification, and immediate feedback for questions
- Enable standardized and convenient training for multiple topics with web-based, userfriendly access to training materials and quizzes
- Allow you to create lab-specific or general quizzes and checklists (e.g., training, fire, OSHA, safety, etc.)
- Automate your competency review process with auto-assignment and defined criteria for recertification (e.g., test a patient, perform QC, perform proficiency testing, quiz)

