The Protecting Access to Medicare Act (PAMA), signed into law in 2014, originated because the Office of Inspector General (OIG) determined that Medicare payments for laboratory tests were often higher than payments for commercial payers. The law’s intent was to update the Clinical Laboratory Fee Schedule (CLFS) and create competitive, market-based laboratory testing prices. However, the data collection methodology used to determine the new rates was flawed, resulting in devastating reimbursement cuts for many labs. The Saving Access to Laboratory Services Act (SALSA) is intended to improve PAMA’s data reporting requirements and payment methodology and thus reduce the negative impact on laboratories.
PAMA
PAMA changed the way that Medicare calculates the CLFS, requiring that Medicare pay the weighted median rate of private payor payments for lab tests. After a confusing collection process, the weighted median became the new CLFS payment rates in 2018. Due to the skewed data collection process, data used to set pricing came from only 1,942 labs out of more than 250,000 labs in the United States, resulting in a poor representation of actual lab fees.
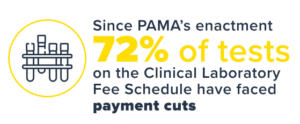
Under PAMA, the first set of Medicare CLFS payment rates resulted in reimbursement cuts for many lab tests—up to 10% each year for 2018, 2019, and 2020. Many commonly performed tests were reduced by 30% during this time. Across the three years of cuts, CLFS lab payments were reduced by nearly $4 billion.1
The LAB Act
In 2019, the Laboratory Access for Beneficiaries (LAB) Act was passed, delaying PAMA’s reporting of lab payment data by one year. The LAB Act also required the Medicare Payment Advisory Commission (MedPAC) to investigate and report a less burdensome data collection process that would include an accurate representative sample of private payer rates from all laboratory types. MedPAC concluded that the original data collection process led to greater cuts than would have occurred if the price reporting had actually represented the laboratory industry as a whole. They proposed a random-sampling approach to collecting lab pricing data that can correct the data collection problem. Their proposal reduces the number of labs required to report and subsequently reduces the overall administrative burden on the lab industry.
Pending Cuts
For 2021 and 2022, PAMA cuts were delayed due to the COVID-19 pandemic. Currently, reductions of up to 15% each year for 2023, 2024, and 2025 remain a possibility under PAMA. Cuts this deep on top of the previous reductions could seriously damage the health infrastructure and Laboratory Response Network.
SALSA
In March of 2023, SALSA was reintroduced with the intention of revamping PAMA’s data reporting requirements and CLFS payment cuts. SALSA will require broader data collection across the full laboratory market and limit annual payment reductions to the CLFS. As MedPAC advised, SALSA requires the Centers for Medicare & Medicaid Services (CMS) to use a statistically representative sample of the entire laboratory market to determine CLFS rates. SALSA also protects against drastic rate cuts, reduces the administrative burden on laboratories (fewer labs are required to report), and increases time between reporting periods from three to four years.
SALSA Overview
- Requires CMS to use a representative sample of the laboratory market to determine CLFS rates
- Reduces the frequency of required data reporting periods from every three years to every four years
- Provides protection against excessive cuts by lowering the cap on cuts from 15% to 5% annually
- Ensures sustainability for the Medicare program by phasing in a 5% cap on yearly rate increases for widely available tests
StopLabCuts.org
PAMA’s reimbursement decreases could have serious consequences for community laboratories, threatening their ability to provide services to the most vulnerable Medicare beneficiaries, in particular those in rural communities, home health care, and skilled nursing facilities.
Clinical laboratories and related groups are actively advocating for SALSA using the Stop Lab Cuts campaign. The Take Action button on the website allows you to easily contact your congressional representatives and let them know that you are concerned about the long-term effect of continued lab testing rate cuts, and that you support SALSA.
The campaign to support SALSA intends to help ensure patient access to laboratory testing and protect the laboratory infrastructure.
Reference
- ACLA.com. ACLA and 25 provider organizations urge Congress to protect access to critical laboratory tests for seniors and all patients. https://www.acla.com/acla-and-26-provider-organizations-urge-congress-to-protect-access-to-critical-laboratory-tests-for-seniors-and-all-patients. Published September 2022.
