While the human genome consists of three billion base pairs of DNA, interestingly, only 0.01 percent varies between individuals. This means that the obvious physical variation seen among people can be attributed to an exceptionally small amount of genetic variance. Yet, this small difference determines our individual traits, predisposition for certain diseases, reaction to environmental factors, and specific response to certain drugs. By performing tests at a molecular level and mapping these differences, we are making huge strides toward improving healthcare and becoming capable of offering more precise and personalized care.
To date, remarkable advances have been made in the science of molecular testing, yet information systems and reimbursement models struggle to keep up. To reap the full benefits that molecular testing has to offer, these systems will need to advance alongside the molecular methodology enhancements. Additionally, as molecular testing advances and is more widely adopted, the lab’s role will change. Automated testing processes mean labs will need to route test results accurately and securely. A shifted focus within the U.S. healthcare industry to value will force labs to have efficient test ordering processes and quick interpretation. Altogether, these changes mean that labs will play a bigger role within the healthcare system.
Executive Summary
- Molecular diagnostics is transforming healthcare by enabling personalized medicine through precise, genetic-based testing and treatment strategies.
- Applications of molecular testing span infectious disease, oncology, pharmacogenomics, genetic screening, HLA typing, and coagulation—supporting diagnostics, treatment selection, and disease monitoring.
- Automation and miniaturization have made molecular testing more efficient, less complex, and more widely available—even in smaller labs.
- Superior sensitivity and specificity of molecular tests enable earlier and more accurate diagnoses, especially for hard-to-culture pathogens and resistant bacteria.
- Companion diagnostics and pharmacogenetics help tailor drug therapies to individual genetic profiles, reducing adverse reactions and improving efficacy.
- Reimbursement remains a major challenge, with evolving CPT and Z-code systems attempting to streamline billing and coverage for molecular tests.
- Precision medicine aims to tailor treatments based on genetic, environmental, and lifestyle factors—supported by national initiatives like the Precision Medicine Initiative (PMI).
- Data management is a challenge, with next-generation sequencing (NGS) generating massive volumes of data that require advanced storage, analytics, and clinical decision support tools.
- The role of the laboratorian is evolving from specimen handling to data interpretation, consultation, and informatics—requiring new skills in IT, automation, and clinical collaboration.
The Human Genome Project
 Following the February 2001 initial draft of the Human Genome Project, on April 14, 2003, the National Human Genome Research Institute (NHGRI) announced the successful completion of the sequencing of the human genome. This ten-year project had a $2.7 billion price tag and spurred thousands of spin-off projects that have rapidly advanced the field of molecular diagnostics. At the time of the project, the predominant methodology was first-generation or Sanger sequencing, a slow, labor intensive methodology developed in 1975 by Edward Sanger, and considered the gold standard for nucleic acid sequencing for the next 25 years.1 Since then, the improvements in molecular testing methodologies have brought the price into the realm of affordable reach; predictably to a day when everyone can afford to have their genome sequenced in the near future.
Following the February 2001 initial draft of the Human Genome Project, on April 14, 2003, the National Human Genome Research Institute (NHGRI) announced the successful completion of the sequencing of the human genome. This ten-year project had a $2.7 billion price tag and spurred thousands of spin-off projects that have rapidly advanced the field of molecular diagnostics. At the time of the project, the predominant methodology was first-generation or Sanger sequencing, a slow, labor intensive methodology developed in 1975 by Edward Sanger, and considered the gold standard for nucleic acid sequencing for the next 25 years.1 Since then, the improvements in molecular testing methodologies have brought the price into the realm of affordable reach; predictably to a day when everyone can afford to have their genome sequenced in the near future.
$1,000 Genome
In 2004, the NHGRI announced the first Advanced Sequencing Technology Awards, otherwise called the $100,000 Genome Grants and $1,000 Genome Grants, to encourage and fund continued growth in sequencing technology, with a goal of attaining a genome sequence for $1,000.2,3
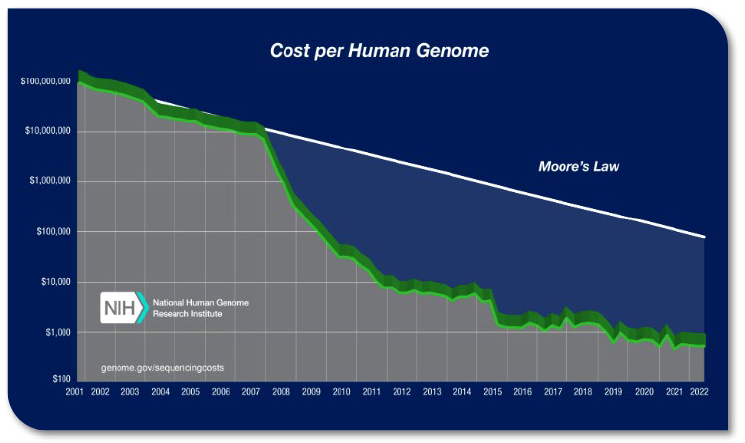
Because of the nuances of cost accounting, it is debated as to whether this cost per test has been truly achieved; nevertheless, gene sequencing costs have dramatically decreased. The rapid decrease in cost has been commonly compared to the slope of Moore’s law, which predicts a biennial doubling of computer processing power. Moore’s law is often used as a comparative measure. Technologies that perform in line with the hypothetical data are considered to be good performers. Beginning in 2007, genome sequencing costs began to dramatically drop, rapidly outpacing Moore’s prediction (see Figure 1).4
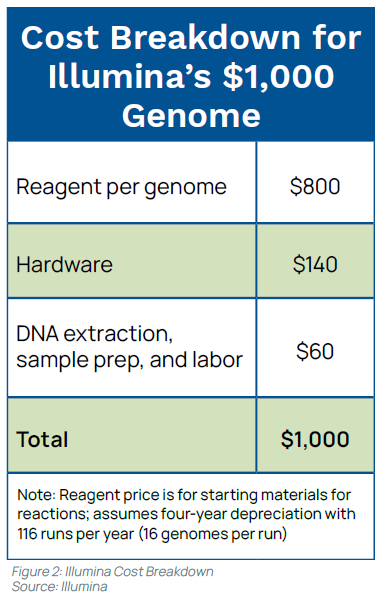
Early in 2014, Illumina announced it had reached the goal using its HiSeq Ten Sequencing System—”the world’s first $1,000 genome,” according to Illumina—that can sequence more than 18,000 human genomes per year for $1,000 each. (see Figure 2).5,6 This trend is expected to continue. Raymond McCauley, Science Officer, Exponential Biosciences at Singularity University, predicts that by 2020 “sequencing a genome will be cheaper than flushing a toilet.”7
Applications of Molecular Testing
The radical shift of focus in our healthcare system has pushed the clinical demand for molecular testing; in response, menus are growing rapidly. The technology originally used for sequencing the human genome, Sanger sequencing, is considered much too complex and uneconomical for laboratories today. Southern blot and Sanger sequencing, widely used 20 years ago, are both very labor intensive and offer low throughput. More automation offers higher throughput, and technology has advanced from single gene tests to panels.
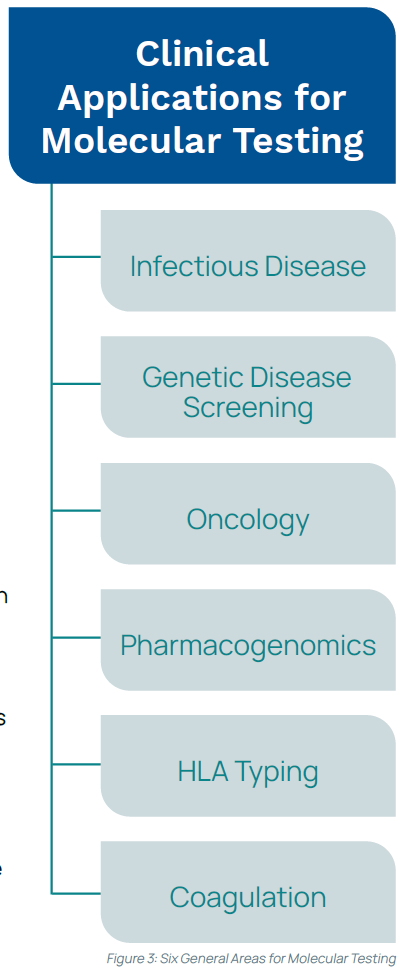
Most clinical applications for molecular testing fall within six general areas: infectious disease, oncology, pharmacogenomics, genetic disease screening, human leukocyte antigen typing, and coagulation (see Figure 3).8 Molecular testing can be beneficial for diagnostics, newborn screening, pre-symptomatic and carrier screening, prenatal diagnosis, prognosis, and personalized or precision medicine. Molecular test results can help a cancer patient understand the likelihood of reoccurrence, aid a provider in selecting the best drug for a specific tumor, or inform a pregnant couple how likely they are of passing on an inheritable gene mutation. Molecular tests can be used to monitor virus levels to determine treatment efficacy, screen donated blood for infectious diseases, identify infectious pathogens, and more.
Infectious Disease Advances
Microbiology has gained enormously from advances in molecular testing in the diagnosis and treatment of infectious diseases. One of the earliest molecular testing successes was in the diagnosis of herpes simplex virus (HSV)-associated encephalitis.9 Shortly thereafter, the U.S. Food and Drug Administration (FDA) approved the first molecular microbiology test to detect Chlamydia trachomatis.
Now we have multiplex polymerase chain reaction (PCR) to detect and differentiate respiratory viruses, such as the BioFire FilmArray Respiratory Panel that can detect 20 respiratory pathogens in about an hour. Several fully automated molecular platforms are available for the rapid diagnosis of infectious diseases.
The alarming rate of hospital-acquired infections (HAIs), now the fourth leading cause of death in the US, is another motivating factor for advancements in infectious disease molecular testing, in particular Methicillin-resistant Staphylococcus aureus (MRSA) and Clostridium difficile. According to the CDC, upwards of two million patients suffer from an HAI every year, and approximately 100,000 of these patients die.10,11 With payments now being tied to performance, reducing HAIs has become a priority. Molecular tests are becoming the solution.
Sepsis, frequently encountered in the ICU and historically difficult to diagnose, has also become an area of focused efforts. Cases increased from 621,000 in 2000 to 1,141,000 in 2008. Sepsis carries a high mortality rate (28-50%), killing more than 258,000 Americans each year, and leaving many survivors with lifelong side effects.12 According to the CDC, sepsis is the ninth leading cause of disease-related death.13 In response, there are now FDA-approved molecular tests for blood culture panels (Verigene, BioFire) that correlate well with culture and sensitivity testing and provide results in just a few hours, a vast improvement over the traditional turnaround time (TAT) associated with culture growth in the microbiology lab.
Successful Molecular Genetic Tests
Molecular genetic testing is available for several applications (e.g., prenatal diagnosis, risk for hereditary cancer, diagnosis of neurologic disorders, and to diagnose/stage malignancies). For example, Myriad Genetics’ BRACAnalysis® uses DNA sequencing to look for inherited mutations in BRCA1 and BRCA2 to determine the risk of developing breast or ovarian cancer. The Genomic Health Oncotype DX® Breast Cancer Assay is used in certain breast cancer patients to predict the benefit of chemotherapy and estimate the risk of reoccurrence, and the Genentech HER2/neu test is used to predict a breast cancer patient’s response to treatment with Herceptin.
 Clinical Exome Sequencing (CES) can sequence 4,800 genes and is used to detect rare genetic disorders. At the 2015 Executive War College, Dr. Gregory Tsongalis, a pathology professor and translational research expert at Dartmouth Hitchcock Medical Center, said that the new highly complex molecular technologies can potentially sequence
Clinical Exome Sequencing (CES) can sequence 4,800 genes and is used to detect rare genetic disorders. At the 2015 Executive War College, Dr. Gregory Tsongalis, a pathology professor and translational research expert at Dartmouth Hitchcock Medical Center, said that the new highly complex molecular technologies can potentially sequence
three billion analytes—a remarkable achievement. “Compared to the DNA sequencers used in the Human Genome Project just two decades ago, today’s technology can produce exponentially more data in a fraction of the time, analyzing each piece of data in a genome repeatedly for unprecedented accuracy,” Tsongalis said.8
Molecular Biomarkers in Cancer
For cancer patients, molecular prognostic (e.g., HER2/neu), or predictive (e.g., eGFR, KRAS) biomarkers provide the clinician with information about the patient’s overall prognosis, or about the efficacy of a treatment plan, respectively.14 Because of the diversity of cancers and the long process for the development of new drugs, the pharmaceutical market is still catching up to the advances in molecular technology.
Molecular Testing at the Point of Care
The rapid TAT offered by point-of-care testing (POCT) has greatly increased the demand for molecular testing at the patient location. In a healthcare environment looking to decrease unnecessary hospital admissions and reduce length of stay, the new methodologies offered in molecular POCT can provide rapid, accurate results to meet these specific needs and make significant financial and patient outcome improvements.
Molecular testing has made big strides in POCT. For example, Enigma Diagnostics’ Enigma ML offers real-time PCR testing with a small footprint. The introduction of single-use, closed systems has created an opportunity for almost any lab to perform molecular testing. Advances have been made in molecular POCT to develop devices that require minimal training and incorporate built-in QC. For many of these devices, the specimen is added to a cartridge and results are available within a few hours.
Having the right POCT in the right location can be of great benefit. For example, Enigma Diagnostics, BioFire FilmArray, and the IQuum Liat Analyzer all offer fast PCR for respiratory viruses, with testing performed one sample at a time.8 Roche acquired IQuum’s laboratory-in-a-tube (Liat™) analyzer, which has automated all aspects of the nucleic acid testing process, including reagent preparation, target enrichment, inhibitor removal, nucleic acid extraction, amplification, and detection.15 Two tests for the Liat, cobas® Influenza A/B, and cobas® Strep A, have recently received FDA-approval and can produce results in about 20 minutes.
The MinION, made by Oxford Nanopore Technologies, literally fits into the palm of your hand (see Figure 4), can reliably sequence small genomes such as bacteria and yeast, and can read portions of the human genome. What is remarkable is that it is inexpensive and plugs into a USB port to display data as it is generated.16
 Less Complex Testing → More Availability
Less Complex Testing → More Availability
The rapid pace of advances taking place in molecular testing is mind-boggling and continues to develop at an extraordinary rate. Testing has become incredibly less complex with much faster TATs available, making molecular testing more widely available and affordable. There are systems that automate all steps of testing, including nucleic acid extraction, amplification, and detection. Advances have been made in large, high-throughput automated systems for high-volume testing that require very little training.
Automation has brought high throughput platforms (e.g., Abbott m2000 RealTime system, cobas 6800/8800 systems, and Qiagen’s QIAsymphony RGQ®). Molecular instrumentation mimics the progression of chemistry instrumentation, and can plug into an automation line. For example, the BD MAX System fully automates cell lysis, nucleic acid extraction, PCR setup, amplification, and detection.17
Superior Sensitivity & Specificity
The superior sensitivity and specificity offered by testing at the molecular level provides accurate and rapid results. Rapid identification of resistant bacteria and measurable correlation to disease severity enables fast therapeutic decisions and early treatments for infectious diseases. Multiplex molecular tests can identify multiple infectious agents in the same specimen,18 and there are new methodologies that can identify organisms that have historically been difficult to culture.19 However, it still remains important, as in any testing methodology, to clearly understand the strengths and weaknesses of each molecular technique so that the most appropriate and cost-effective method is selected for economic efficiency and analytical validity.
 Aid in Developing More Personalized Treatment Plans
Aid in Developing More Personalized Treatment Plans
An individual’s genetic makeup and their surrounding environment can greatly influence the development of illness and a person’s response.20 By testing and understanding an individual’s unique genetic profile and their susceptibility to certain diseases, care providers can begin to delve into the science of personalized medicine.
For certain medications, physicians can use molecular diagnostic laboratory tests to determine the benefits and potential side effects for an individual, referred to as companion diagnostics. Companion diagnostics associates the patient’s molecular or genetic makeup with how they will metabolize a particular drug. This can detect which patients are likely
to benefit from a specific drug and which may be at risk for an adverse drug reaction or help determine optimal dosages and treatment frequencies.
Challenges
While the cost of molecular testing is quickly dropping into a more attainable range, start-up costs for next-gen sequencing (NGS) range between $100,000 to $150,000, still a price that many labs cannot afford.21 This is especially true in light of the reimbursement confusion that surrounds molecular testing. Health insurance reimbursements for molecular diagnostic tests are a detriment to more widespread adoption, as this landscape is conflicting and confusing. Disease-targeted NGS panels are more likely to be covered by payers than entire genome sequencing, and there is still debate over the potential for unintended consequences from whole gene sequences.
Molecular Reimbursements
Technological advancement has happened at a faster pace than pharmaceutics and lab reimbursements have been able to match. Both public and private payers have struggled to keep up with the advances in molecular testing, resulting in a complex and frustrating reimbursement system.
The historical and inadequate approach for molecular reimbursement involved using CPT codes that coded for the steps of the process (e.g., isolation, amplification, enzymatic digestion, separation), called “stacking codes.” This method often resulted in denials or low reimbursements because it did not accurately identify the tests.
Having the codes based on the method or step being performed rather than on the analyte made it nearly impossible for payers to properly identify and reimburse for molecular testing.
CPT codes are now evolving and improving, but the pace, but the pace of change is very slow. In 2012, the American Medical Association (AMA) introduced an analyte-specific, tiered coding system for molecular pathology (called MoPath codes) to replace the subpar methodology-based stacking codes. MoPath codes allow for more accurate labeling and identification of molecular diagnostic tests, which in turn helps payers properly identify and bill for testing. In 2013, labs began using the MoPath codes and stacking codes were discontinued.22
 MoPath codes are categorized into Tier 1 and Tier 2 codes. Tier 1 codes are the commonly performed single-analyte molecular tests, and represent about 90% of the codes. Tier 2 codes are used for lower volume tests, and are broken down into nine levels that represent the amount of technical and interpretive effort involved.22
MoPath codes are categorized into Tier 1 and Tier 2 codes. Tier 1 codes are the commonly performed single-analyte molecular tests, and represent about 90% of the codes. Tier 2 codes are used for lower volume tests, and are broken down into nine levels that represent the amount of technical and interpretive effort involved.22
Palmetto GBA, a CMS contractor, introduced the MolDX Program in 2011 to begin registration of molecular tests in order to identify available testing and determine clinical utility of molecular tests. This information was to be used to improve claims processing and reimbursement.23 Meanwhile, also in 2011, McKesson created the McKesson Diagnostics Exchange™ (DEX), to allow manufacturers to submit and catalog information about their specific molecular tests. Providers and payers use this index to evaluate the tests, tracking outcomes and analyzing the clinical utility of each test.24 The DEX assigns unique Z-Code Identifiers to each test and catalogs it for reference. In 2014, McKesson and the AMA began collaborating to group and index the Z-Codes with corresponding CPT codes to improve and simplify the reimbursement process.24
The pace of being assigned a Tier code has been slow because gathering the evidence needed to support a new code is much slower than the rapid pace of new molecular discoveries. In the meantime, the stepping stone is Z-Code Identifiers, which are unique identifier tracking codes that are not yet CPT codes. Both codes are required on a claim. As of the end of 2014, there were about 4,500 Z-Codes, with only about 500 identified as Tier 1 or 2 tests because of the slow process of gaining enough evidence to assign a CPT code. For example, there may be multiple versions of the same molecular test, with unique Z-Code Identifiers, but with minor differences in test methodology.24
In 2022, reimbursement rules were issued for molecular syndromic panels, defining a syndromic panel as a test that simultaneously detects multiple pathogens, and dividing syndromic panels into “targeted” (tests for five pathogens or less) and “expanded” (tests for six pathogens or more). Effective in 2023, UnitedHealthcare Services updated its reimbursement policy on molecular pathology testing, requiring submission of a DEX Z-Code with every molecular pathology testing code. The trend over the last decade has been to add more structure around billing for molecular testing, with increased emphasis on the use of DEX Z-Codes to improve transparency and streamline the reimbursement process.
As the process continues, the dilemma is not only how much an insurer will pay, but will they pay at all. Coverage determinations remain difficult to obtain. Payers are looking for clear evidence to support coverage of a test including: analytical validity, clinical validity, and clinical utility. Clinical utility, which refers to how much a test influences clinical decision-making or improves patient outcomes, has become the most difficult to measure and prove and, consequently, the most important to payers in determining coverage. Palmetto GBA requires clinical utility studies before evaluating a test for coverage under the MolDx program.22
Shared Gene Databases
In an effort to adequately store and share genetic data, databases of genetic variants and how they relate to disease have built up significantly in recent years, dramatically advancing the clinical utility of genetic data. As labs around the world share this information, scientists, clinicians, and patients everywhere get the benefit of more accurate genome interpretations.8
Sequencing labs submit their data to big archival databases (see list below) who maintain, organize and distribute the data.30
- GenBank® at the National Center for Biotechnology Information (NCBI)
- European Bioinformatics Institute EMBL database
- DNA Data Bank of Japan (DDBJ)
- Sequence Read Archive (SRA)
- Gene Expression Omnibus (GEO)
- ArrayExpress
The Cancer Genome Atlas (TCGA) is a U.S. project started in 2005 to catalog cancer-causing mutations. TCGA has been instrumental in developments for new cancer treatments (e.g., Gleevec for GI stromal tumors).29
1000 Genomes, not to be confused with the $1,000 genome, is NHGRI funded; it directs the 1000 Genomes Project, the world’s largest catalog of human genes, as a resource for understanding disease.32, 33 This resource compiles emerging research on the molecular makeup of diseases from individual patient data to promote advancements in disease classification and improve future diagnosis and treatment.
 Moving Toward Precision Medicine
Moving Toward Precision Medicine
The terms personalized medicine and precision medicine are often used interchangeably. Personalized medicine is an older term that means identifying the genetic or molecular basis of a patient’s disease along with their specific individual genetic makeup to target the best course of therapy.35 While precision medicine means close to the same thing, it is a newer, broader term that refers to the tailoring of treatment to the individual characteristics of each patient. Think of precision medicine as the overall approach to developing treatments with better outcomes based on clinical and molecular information and personalized medicine as the treatment decisions that result from this information.
Precision medicine classifies patients into subpopulations based on genes that influence susceptibility to diseases, prognoses, and responses to specific treatments. From this, specific treatments can be targeted toward populations that are likely to benefit, rather than providing a treatment that may have undesirable side effects in patients who are not likely to respond. According to the National Institutes of Health (NIH), precision medicine is “an emerging approach for disease treatment and prevention that takes into account individual variability in genes, environment, and lifestyle for each person.”35
Providers use precision medicine data to improve treatment outcomes for their patients, but this does not necessarily mean creating a drug specific to one patient. Significant advances in precision medicine have been made for many cancers, but precision medicine is not currently used for most diseases. Precision medicine is beginning to be desirable in healthcare. To accelerate the pace of precision medicine research and adoption, in 2015 President Barack Obama announced the Precision Medicine Initiative (PMI), intended to “revolutionize medicine and generate the scientific evidence needed to move the concept of precision medicine into every day clinical practice.”42 In his January 20th, 2015 State of the Union Address, President Obama said, “I want the country that eliminated polio and mapped the human genome to lead a new era of medicine—one that delivers the right treatment at the right time… I am launching a new precision medicine initiative to bring us closer to curing diseases like cancer and diabetes—and to give all of us access to the
personalized information we need to keep ourselves and our families healthier.” 42
Precision medicine has been referred to as the future of medicine and is meant to consolidate and analyze worldwide genetic data, clinical information, and environmental and lifestyle data; to look for insights into the root cause of disease and thereby promote more targeted and successful treatments. The PMI targets $215 million to study genome data, health records, and physiological measurements from one million participants to learn how genetics, environment, and lifestyle influence disease risk and treatment efficacy.33
 Companion Diagnostics
Companion Diagnostics
Precision medicine and personalized medicine go hand-in-hand with companion diagnostics. Genetic and molecular laboratory tests are used to determine the benefits and risks associated with taking certain medications. Knowing how a patient will metabolize a certain drug can help determine which patients will benefit most from a drug and which may be at risk for adverse events. Companion diagnostic tests can help determine the optimal dosage to achieve the best outcome based on a patient’s genetic profile.
Companion diagnostics have developed most quickly in the specialty of cancer in the quest for more effective, targeted therapies. By analyzing the molecular reactions of patients with cancer, providers can evaluate drug effectiveness. As physicians learn more about their patients’ DNA, new drugs are being developed that treat diseases at the molecular level.
The combination of drug development and diagnostic testing is called pharmacogenetics or pharmacogenomics and feeds directly into precision medicine.
Pharmacogenomics/Pharmacogenetics
Each individual has a specific genome that reacts to drugs differently based on their genetic makeup. The study of these genetic variations and their relationship to drug response is called pharmacogenetics, and is closely related to precision medicine. The goal of personalized medicine is to individualize healthcare by using knowledge of patients’ health history, behaviors, environment, and genetic makeup when making clinical decisions.
The terms pharmacogenomics and pharmacogenetics, while often used synonymously, are slightly different. Pharmacogenetics involves looking at an unexpected or adverse drug response and trying to determine a genetic cause, whereas pharmacogenomics is looking for genetic differences within a population to explain actual responses to a drug or susceptibilities to a disease.43 Another way of explaining it is that pharmacogenetics is viewed from the perspective of individual genes; pharmacogenomics is a broader term that implies a genome-wide approach to studying the body’s response to drugs.44
Molecular Profiling of Tumor Cells
From a molecular standpoint, not only are no two people the same, but neither are two tumors. Discovering the exact DNA sequence of a patient’s tumor can point doctors to the best treatment and allow for better tracking of treatment efficacy. With cancer, the individual genome has to be taken into account as well as the genome of the cancer cells or tumor tissue. Treatments can be tailored to specific mutations unique to the tumor cells. In order to accomplish this, molecular profiling of the tumor has to happen to select the most effective treatment plan.
An early example of targeted therapy was using Gleevec to treat chronic myelogenous leukemia (CML). Identifying the BCR-ABL protein in Philadelphia-positive CML cells meant that the Gleevec would be beneficial to the patient. This technique greatly improved the five-year survival rate for patients with CML, and looks promising for more than 500 other targeted therapies.37
Biggest Challenge: Handling the Data Deluge
Since its inception, NGS data output has more than doubled every year. From 2007 to 2012, the amount of data produced from one sequencing run has increased from one gigabase (Gb) to one terabase (Tb).21 The difficulty is no longer in test performance, but in the interpretation of large, complex data sets.
 With sequencers producing up to 0.5 terabases per device per day, a huge challenge exists to store, manage, and share data. The ability to compute and organize a patient’s entire genome is still lacking because of the issues involved in processing the data. Mountains of genomic data are accumulating that are of little use because they are not tied to clinical information, such as family medical history. Often, genomic data are in documents that cannot easily be searched, shared, or understood by most physicians.28
With sequencers producing up to 0.5 terabases per device per day, a huge challenge exists to store, manage, and share data. The ability to compute and organize a patient’s entire genome is still lacking because of the issues involved in processing the data. Mountains of genomic data are accumulating that are of little use because they are not tied to clinical information, such as family medical history. Often, genomic data are in documents that cannot easily be searched, shared, or understood by most physicians.28
As molecular methods become more sophisticated, so does the need for advanced reporting and interpretative tools, and storage of the large amounts of data generated. Genomic data needs to be catalogued and used to advance precision medicine.29 The cost of genome sequencing is now decreasing several times faster than the cost of storage, promising that at some time in the not too distant future it will cost less to sequence a base of DNA than to store the resulting data.30
The future of personalized medicine depends on being able to perform robust analytics that lead to evidence-based recommendations. We will have to perform computational modeling to make valid predictions, and incorporate information from the medical literature that leads to an understanding of how a genetic variant will affect a patient’s treatment.19
Armstrong (2012) posits that there are two distinct requirements for the information technology of the future in regards to genetic testing: adequate storage capabilities that allow for future access, and appropriate clinical decision support to guide providers in the best use of genetic tests.31 To achieve the full benefit offered by molecular and genetic testing, IT vendors need a clear focus on the technologies necessary to accomplish these goals.
How Do Advances in Molecular Testing Change the Role of the Laboratorian?
Lab testing, concurrent with other medical specialties, has steadily moved from being a very hands-on process to becoming more automated. The majority of large laboratories now include automation lines that handle the routing of samples, and automation is expanding its reach into microbiology, anatomic pathology, and molecular testing (e.g., DNA extractors).38
Furthermore, the role of the laboratorian of the future is expected to become more consultative. For example, providers are demanding additional support in the interpretation of genetic test results.39 The lab will be involved in using software solutions to sort through and analyze the large amounts of data generated by genetic testing. We are looking at a future where IT systems can analyze databases with genome analyses from hundreds of thousands of patients for trends or connections that help determine the best treatment plan for a particular patient.40
So, how will this future look for the laboratorian? With IT advances, molecular and genetic testing advances, combined with the changing healthcare environment, the lab is transitioning even further away from hands-on processes to a reality where the specimen is rarely touched. What the laboratorian will “touch” is the data—being involved in valuable interpretations, test consultations and algorithms, and lab informatics. The National Accrediting Agency for Clinical Laboratory Sciences (NAACLS) predicts an expanding role for the laboratorian.

Among other new duties, NAACLS purports that laboratorians will be more involved in consultative services, interpretative guidance, evaluation of patient outcomes, clinical information system teams, working with genetic counselors, automation engineering, and IT engineering as it relates to the lab.41 Future generations of laboratorians will be expected to be involved in molecular informatics, take a bigger role on the healthcare team, have advanced lab IT skills, and be involved in projects outside of the laboratory.
If you think about the fact that Sanger sequencing was developed in 1975, the entire genome was sequenced in 2003, the first moderately complex molecular test was developed in 2006, and the first CLIA-waived molecular test approval was in 2015 (Alere i Influenza A&B);42 the molecular industry’s pace is exponentially accelerating. We are looking into uncharted territory, where genetic sequencing is routinely performed and we use molecular lab data to quickly and accurately assess a patient’s health. These advances in research and treatment are transforming the practice of medicine and the laboratory plays an important role in the transformation.
Molecular Diagnostics and the Lab’s Expanding Role in Personalized Medicine
We are at an unprecedented place in medicine with advances in molecular testing, creating a vast potential to make positive changes within our healthcare system. Never before have providers had access to the tools, technology, and data that we now have to begin to customize diagnoses and treatments for patients.
The laboratory is the key to diagnostics, and the role of the laboratory is rapidly evolving. The lab is transitioning from being the stewards of specimens to being the purveyors of data. By gaining a better understanding of genomic data and using that information to its full potential, the implications for improvements in the way we practice medicine offer unlimited possibilities. Molecular diagnostics is transforming and revolutionizing healthcare by paving the way for tailored treatments at the individual level—personalized medicine. With healthcare reform placing greater emphasis on prevention and taking into account the influence from our environment, diet, and lifestyle, molecular testing can change the future of healthcare in ways we have not even thought of yet. Laboratory professionals must engage in active education with other healthcare colleagues to promote the integration of molecular advances into routine patient care;9 and aid in molecular data analysis and result interpretation to realize the full benefit of molecular testing and promote lab value.
Orchard® Molecular™ includes dynamic workflow support that orchestrates the entire molecular testing process from pre-analytical through post-analytical. Our systems capture data in a searchable, integrated format and offer a flexible implementation, allowing Orchard Software to meet the connectivity, workflow, and reporting needs of molecular laboratories.
| Definitions | |
Companion Diagnostics |
Genetic and molecular diagnostic laboratory testing used to determine the benefits and potential side effects for an individual |
DNA Polymerase |
A naturally occurring complex of proteins whose function is to copy a cell’s DNA before it divides in two |
Exome |
Protein-coding regions of the genome; comprises just over 1% of the genome and is therefore more cost-effective to sequence than the entire genome |
Genetic Test |
A test that analyzes various aspects of an individual’s genetic material (DNA, RNA, chromosomes, and genes); All genetic tests are IVDs. Most genetic tests are LDTs. |
Genomics |
The study of all the genes in an organism; activities and interactions of genes |
Gigabase |
One billion bases (nucleotides) as a unit of length of a nucleic acid |
Nucleotides |
Building blocks of DNA molecules; the four types of nucleotides are Adenine, Cytosine, Guanine, and Thymine |
Polymerase Chain Reaction (PCR) |
Methodology that uses DNA polymerase to synthesize a new strand of DNA complementary to the template strand; at the end of the PCR reaction, the specific sequence will be accumulated in billions of copies (amplicons) |
Pharmacogenetics (PGx) |
Combining pharmacology and genetics; the study of how a genome metabolizes drugs |
Pharmacogenomics |
Study of genetic differences within a population to explain observed responses to a drug or susceptibility to a disease |
Primers |
Short pieces of DNA that are developed in a laboratory; primers can have any sequence of nucleotides |
Proteomics |
The study of proteins made by genes |