Tools for Workflow Efficiency, Patient Safety, Medication Adherence, Faster Turnaround Times, and Integrated Result Reporting
Abstract
Toxicology labs face unique challenges in sample collection requirements, regulatory compliance, reporting, and billing practices, each of which can be improved upon with a toxicology-focused LIS. Orchard Software’s toxicology-focused LIS is designed to manage the specific needs of toxicology labs and help address industry challenges.
Each laboratory specialty has unique challenges. For toxicology laboratories, one such challenge is the close monitoring of billing practices. The Office of Inspector General (OIG) released a report stating that between 2016 and 2020, Medicare paid $704 million for definitive drug testing for which medical necessity was in question.1 Billing challenges top a list of several other intricate areas of responsibility within toxicology labs. The good news is that by leveraging the tools and automation of a toxicology-focused laboratory information system (LIS), many of the top challenges can be more easily managed.
Executive Summary
Toxicology testing includes both therapeutic drug monitoring (TDM) and drug of abuse testing (DAT).
Toxicology labs face unique challenges in sample collection requirements, regulatory compliance, and reporting and billing practices—each of which can be improved upon with a toxicology-focused LIS.
Orchard Software’s toxicology-focused LIS is designed to manage the specific needs of
toxicology labs and help address industry challenges.
 Toxicology Lab Overview
Toxicology Lab Overview
Clinical toxicology laboratories are specialized labs that focus on drug testing—identifying chemicals, toxins, and drugs. Drug testing can be used to determine the presence or absence of drugs or drug metabolites and can be quantitative or qualitative depending on the testing purpose.
Toxicology testing includes both TDM and testing for inappropriate drug use. TDM measures drug levels to optimize dosing and to assess patient compliance with their medication schedule. Clinical toxicology labs perform testing for patients in pain management or substance abuse programs to ensure patients are taking the medications they are prescribed, and not taking medications that they are not prescribed.
DAT is lab testing for the use of illicit drugs such as cocaine or phencyclidine and potentially addictive therapeutic medications such as benzodiazepines, opiates, or amphetamines. For DAT, often, quantitative levels are not required. Laboratory testing reveals if a drug is present or absent based on a pre-defined cutoff concentration.
For positive screening samples, labs use confirmatory testing to identify the drug or metabolite. Confirmatory testing is expensive and has a longer turnaround time (TAT), but is required for forensic, legal, and pre-employment testing that mandates quantitative results.
Toxicology Lab Challenges
Toxicology laboratories face many of the same challenges as other clinical labs, such as recruiting and retaining well-trained staff and maintaining acceptable TATs. However, toxicology labs also have unique challenges with sample collection requirements, regulatory compliance, and billing practices. A strong toxicology-focused LIS can help improve each of these areas.
Challenge #1: Complying with Regulations
The type of accreditation required by a toxicology laboratory depends on the purpose of the laboratory’s testing menu. Like all clinical laboratories, clinical toxicology laboratories are subject to Clinical Laboratory Improvement Amendments (CLIA) inspections and are classified as waived, moderate, or high complexity.
 Forensic toxicology laboratories are CLIA-exempt but are subject to applicable state and local regulatory requirements, which typically mandate independent laboratory accreditation. Accreditation is provided by the College of American Pathologists, American Board of Forensic Toxicology, American Society of Crime Laboratory Directors, and Substance Abuse and Mental Health Services Administration National Laboratory Certification Program.2
Forensic toxicology laboratories are CLIA-exempt but are subject to applicable state and local regulatory requirements, which typically mandate independent laboratory accreditation. Accreditation is provided by the College of American Pathologists, American Board of Forensic Toxicology, American Society of Crime Laboratory Directors, and Substance Abuse and Mental Health Services Administration National Laboratory Certification Program.2
In 1975, the Department of Transportation (DOT) guidelines for workplace drug testing required specific drug testing for new hires with defined cutoffs and that testing be performed by a certified toxicology laboratory. These cutoffs were adopted by other laboratories. In 2017, the DOT established 49 CFR Part 40 that updated guidelines for laboratories.3 Laboratories that participate in DOT testing are required to be certified by the Department of Health and Human Services under the National Laboratory Certification Program. Toxicology laboratories must remain aware of, and comply with, the various regulatory elements surrounding drug testing.
Challenge #2: Collecting Viable Samples
Similar to other lab specialties, specimen collection is vital when testing toxicology specimens. When performing DAT, toxicology labs must be wary of sample tampering. For example, urine is easy to collect but subject to adulteration that can produce false negative results. Various household chemicals such as vinegar and sodium bicarbonate could be added in an attempt to cause false negative results. Many of these additives change the pH of the sample, so laboratories must test the samples’ pH.
Depending on the testing purpose, a toxicology lab may need to comply with chain of custody (CoC) collection procedures. CoC is used to document that the sample collection was overseen by approved personnel and there was no opportunity for tampering.
 Challenge #3: Evolving Testing to Stay Up to Date
Challenge #3: Evolving Testing to Stay Up to Date
New generations of drugs, new therapies, and expanded uses of drug classes make it challenging for toxicology labs to align their testing methodologies and stay current. Prescribing trends and drug abuse patterns are continually evolving, making it difficult for toxicology labs to keep up with the available immunoassays.
The rapid emergence of new psychoactive substances (NPS) adds a level of complexity to toxicology testing. NPS may be developed without quality or regulatory guidelines, making their composition unknown. By the time the toxicology research is complete on a NPS, it may be further modified. Labs may struggle to detect novel synthetic drugs with the available testing methodologies.5
To help address this challenge, clinical toxicology laboratories need to remain nimble, have robust assays, and adjust their testing menus regularly. Mass spectrometry assays designed around a targeted list of drugs with lower concentration
cutoffs, or those that provide quantitative results, fit this purpose. Because mass spectrometry is flexible, clinical laboratories can design their assay to contain the specific drugs of interest to their clinicians and patients.
 Challenge #4: Meeting Billing Requirements
Challenge #4: Meeting Billing Requirements
Medical billing for toxicology laboratories is complex and subject to frequent updates. Toxicology test billing is closely scrutinized by the Centers for Medicare & Medicaid Services (CMS), the OIG, and the U.S. Department of Justice.
Medicare implemented G-codes in 2015 that were intended to simplify billing, which most insurance companies followed. Then in 2016, Medicare introduced bundled coding (G0480-G0483) for definitive drug testing.
For 2023, CMS has asked Medicare Administrative Contractors (MACs) to focus payment audits and claim reviews on labs performing definitive drug testing. This initiative is part of the CMS Targeted Probe and Educate (TPE) program intended to help identify and correct common billing errors. Toxicology billing requirements mandate strict medical necessity documentation. Lack of complete documentation is the most common reason for denials.7
To ensure labs receive appropriate reimbursement for toxicology testing and do not make billing mistakes that attract the unwanted attention of regulators, toxicology labs must stay informed on the guidelines, which frequently change.
In addition to national coverage determinations regarding how drug testing is covered by Medicare, toxicology testing labs must comply with local coverage determinations (LCDs). LCDs are specific billing rules that mandate under what circumstances a particular test will be covered and set the rules for the MAC regions.
LCDs have strict medical necessity rules and specific documentation requirements. For example, definitive drug testing is only indicated when:
- Screening test results are presumptively positive
- Screening test results are negative and inconsistent with the patient’s medical history
- When the coverage criteria of the policy are met and there is no presumptive test available, either locally and/or commercially, as may be the case for certain synthetic or semi-synthetic opioids8
Newly proposed LCDs for several MACs intend to limit reimbursement for definitive drug testing to no more than 14 drug classes.
 Challenge #5: Including Interpretation Guidance on Reports
Challenge #5: Including Interpretation Guidance on Reports
Physicians—especially those outside of pain clinics and treatment centers—often struggle with interpreting toxicology reports. Understanding why and when a test result may yield a false positive or negative, and knowing the appropriate follow-up actions, can be a challenge for these practitioners. A patient’s drug level concentration means little without the context of what medication levels are expected and are desirable. This means laboratories are responsible for providing a report that physicians can easily interpret.
Comprehensive reporting with interpretive comments can help both patients and providers overcome challenges related to interpreting toxicology testing results, especially for those who are less familiar with these types of results.9
 Challenge #6: Finding a Toxicology-specific LIS Solution
Challenge #6: Finding a Toxicology-specific LIS Solution
It can be difficult for a toxicology lab to reach its full potential without an information management solution designed for the specific work being performed. To handle these challenges, clinical toxicology labs can benefit by implementing an LIS that is specifically focused on toxicology and by working with a vendor that is actively engaged in developing toxicology-focused LIS functionality. Investing in a toxicology-specific LIS helps address the advanced technology needs, workflows, and business situations of toxicology labs.
 Tackle Your Lab’s Challenges with Orchard’s Toxicology LIS Software
Tackle Your Lab’s Challenges with Orchard’s Toxicology LIS Software
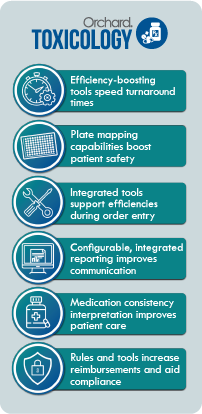
Orchard Software proactively researches the changing toxicology landscape to better support the laboratories that we serve. Orchard’s toxicology-focused LIS—Orchard® Enterprise Lab™ Toxicology module—is designed to manage the specific needs of toxicology labs and help address industry challenges.
Partnering with Orchard Software gives your lab the ability to grow your compliance strategy, increase your workflow efficiencies, and ultimately improve your revenue.
Support for Regulatory Compliance
A toxicology-focused LIS is a long-term solution for labs needing to stay updated on the numerous state and federal regulations. Orchard Enterprise Lab Toxicology module uses data mining tools and decision-support rules to standardize processes and make compliance easier for toxicology labs. Orchard’s solutions allow technologists to easily monitor and document compliance activities.
Flexible Tools for Lab Efficiency & Safety
For sample processing, Orchard’s toxicology solution allows your lab to customize order choices specific to each ordering location. In the analytical phase of testing, the solution provides automated reflex confirmatory testing based on screening results and medications. Users can associate medications with aliases to identify generic or street names and map to the appropriate analyte.
Tools that Help Track Reimbursement Requirements
For reimbursement support, Orchard Enterprise Lab Toxicology module can keep track of LCDs with medical necessity and testing frequency checks. The LIS also includes decision-support logic for prior authorization; rules can be set up to require an authorization number in a user-defined field at order entry.
Configurable Reporting Options to Aid Interpretation
Orchard’s solutions offer flexible report options and user-friendly interfaces to make it easy to create useful toxicology reports. Report setup can be configured by your lab without needing to contact Orchard; each ordering location can have a customized patient result report format to meet your clients’ preferences. Orchard’s solutions provide the ability to track medication and metabolite relationships to indicate consistency with patient prescribed medications and alert the provider of inconsistencies on the report.
 Orchard Toxicology
Orchard Toxicology
Orchard’s toxicology-focused laboratory information solution, Orchard Enterprise Lab Toxicology module, provides an integrated workflow engine for increased efficiency, plate mapping tools that improve patient safety, and medication consistency interpretation that enables providers to improve patient care. With Orchard’s toxicology-focused LIS, labs can improve patient care for their clients by providing tools that drive workflow efficiencies, patient safety, medication adherence, faster TAT, and integrated result reporting. Call us to learn more about Orchard’s toxicology LIS solutions.
